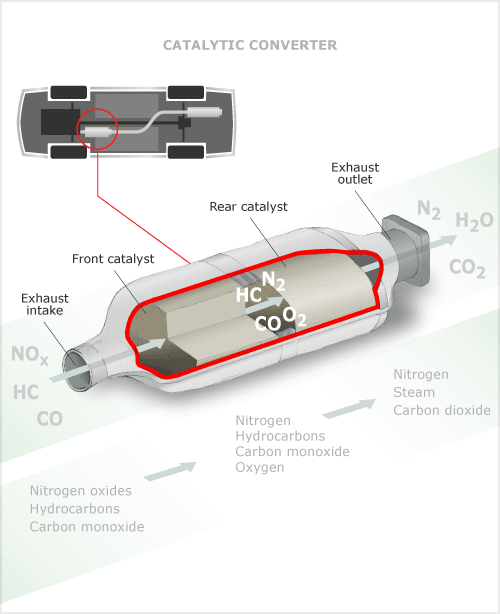
Carbon monoxide poisoning from car exhausts was a significant method of suicides in the 1990s. The spread of catalytic converters, which became compulsory in imported cars, was responsible for reducing the incidence of such poisoning by half between 1997 and 2007. This diagram shows how catalytic converters work. The converter is placed in the exhaust system close to the exhaust manifold. Gases dangerous to both humans and the environment – such as carbon monoxide, hydrocarbons and nitrogen oxide – enter the converter from the vehicle's engine. Within the converter a honeycomb grid coated with platinum, rhodium or palladium then converts the gases into the relatively harmless exhausts of water or steam, carbon dioxide and nitrogen.
Using this item
Te Ara - The Encyclopedia of New Zealand

This item is licensed under a Creative Commons Attribution-NonCommercial 3.0 New Zealand Licence





Add new comment